TOKYO, September 8, 2025 - (JCN Newswire) - Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that the results from a Phase Ib clinical trial (NCT06462404) of its in-house developed novel selective orexin 2 receptor agonist E2086 have been presented at the World Sleep 2025 congress, held in Singapore from September 5 to 10. These data demonstrate that once-daily dosing of E2086 has the potential to improve daytime wakefulness in individuals with narcolepsy type 1 (NT1).
Clinical Trial Design
This randomized, double-blind, single-dose, multiple crossover, Phase Ib clinical trial was conducted in the United States and Canada to evaluate the efficacy, safety, and tolerability of E2086 in people meeting criteria for NT1, compared with placebo and an existing treatment (modafinil). There were a total of 21 patients in the clinical trial (21 patients received the study medication, 19 patients completed the trial). Participants received a single dose of one of five treatments (E2086 5 mg, 10 mg, 25 mg, placebo, ormodafinil) shortly after waking, efficacy assessments were conducted for each treatment.
Efficacy was assessed using the objective Maintenance of Wakefulness Test (MWT)*1 to evaluate excessive daytime sleepiness (EDS), and subjective sleepiness was measured using the Karolinska Sleepiness Scale(KSS)*2 at the end of each MWT session.
Clinical Trial Results
As evaluated by MWT, all doses of E2086 demonstrated statistically significant longer sleep latencies*3 – a measure of the ability to stay awake – compared with placebo (P<0.0001 for all doses) and modafinil (E20865 mg: P=0.0009; E2086 10 mg and 25 mg: P<0.0001) (See the figure below).
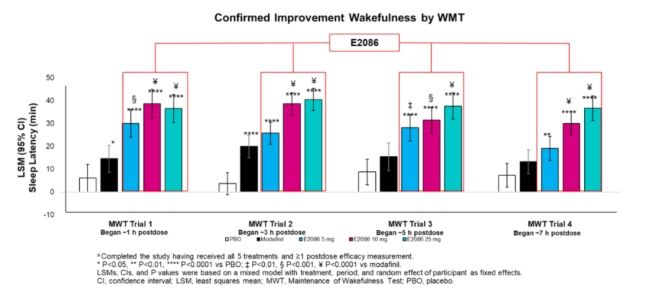
For the KSS, all doses of E2086 showed statistically significant higher alertness compared with placebo(P<0.0001), while E2086 10 mg and 25 mg demonstrated statistically significantly higher alertness compared with modafinil (P<0.0001). These outcomes confirmed the Proof of Mechanism for E2086.
Treatment-emergent adverse events (TEAEs), based on the 21 patients who received the study medication in the clinical trial, exhibited a dose-dependent trend. The four most frequently observed TEAEs with E2086 included increased urinary frequency (E2086 5 mg: 14.3%, 10 mg: 23.8%, 25 mg: 52.4%), nausea (14.3%,19.0%, 47.6%), dizziness (4.8%, 23.8%, 38.1%), and urinary urgency (9.5%, 19.0%, 38.1%). No participants discontinued due to TEAEs, and most TEAEs were mild to moderate in severity. No serious TEAEs were reported.
Katsutoshi Ido, Ph.D., Chief Scientific Officer at Eisai, commented: “At World Sleep 2025, we presented data supporting the potential of E2086 to improve daytime wakefulness in people diagnosed with narcolepsy type 1. Narcolepsy is a condition with high unmet medical needs, characterized not only by excessive daytime sleepiness but also by symptoms such as cataplexy, hypnagogic/hynopompichallucinations, and sleep paralysis, all of which significantly impair the quality of life of those affected. The efficacy and safety findings from this trial warrant further investigation in the narcolepsy patient population.”
Orexin is a neurotransmitter that plays a central role in regulating sleep and wakefulness. Inhibiting orexinergic neurons is believed to promote a natural transition from wakefulness to sleep. Conversely, activating orexinergic neurons is believed to help maintain a more stable state of wakefulness. From the perspective of inhibiting orexinergic neuron activity, Eisai has developed the insomnia treatment DAYVIGO® (generic name: lemborexant) an orexin receptor antagonist that is available in more than 25 countries worldwide. Furthermore, leveraging the orexin platform established through the development of DAYVIGO, Eisai has created E2086, an orexin receptor agonist that activates orexinergic neurons. In addition to DAYVIGO, Eisai aims to contribute to people living with sleep disorders through the development of E2086.
*1 Maintenance of Wakefulness Test (MWT): An objective test that evaluates the ability to stay awake in a sleep-conducive environment. The test is conducted in a quiet examination room with the lights turned off, consisting of four 40-minute sessions carried out at two-hour intervals throughout the day, during which the time taken to fall asleep (sleep latency) is measured using electroencephalography.
*2 Karolinska Sleepiness Scale (KSS): A simple subjective scale used to assess the current intensity of sleepiness ona 9-point scale, ranging from "1 - Very clearly awake" to "9 - Very sleepy, struggling against sleepiness”.
*3 Sleep latency: The amount of time it takes to fall asleep.
For more details, please visit:
https://www.eisai.com/news/2025/pdf/enews202562pdf.pdf
MEDIA CONTACTS:
Eisai Co., Ltd.
Public Relations Department,
TEL: +81-(0)3-3817-5120
Eisai Europe, Ltd.
EMEA Communications Department
+ 44 (0) 797 487 9419
Eisai Inc. (U.S.)
Libby Holman
+ 1-201-753-1945