TOKYO, Sept 30, 2025 - (JCN Newswire) - Olympus Corporation (Olympus), a global MedTech company committed to making people’s lives healthier, safer and more fulfilling, today announced the commercial launch of its OLYSENSE™ CAD/AI (artificial intelligence-powered computer-aided detection) portfolio in the United States and European markets. This portfolio uses AI to enable earlier detection, enhance clinical outcomes, and ultimately improve patient care. In European markets, the launch includes the clinical applications CADDIE™, CADU™, and SMARTIBD™. In the United States, the launch includes CADDIE™ with detection capabilities only.
With more patients needing endoscopic evaluation due to demographic trends and ubiquitous lower and upper gastrointestinal conditions, demand for procedures continues to rise. Yet gastroenterologists must manage heightened workloads, administrative burdens, budget constraints, and staff shortages. AI-based and data-driven solutions offer opportunities to address these demands, supporting efficient screening, earlier diagnosis, and helping care teams focus on appropriate treatment and, potentially, better outcomes.
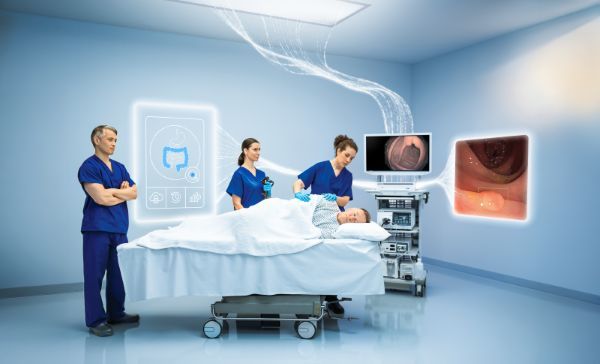 Image of OLYSENSE in an endosuite1
Image of OLYSENSE in an endosuite1
Olympus has introduced OLYSENSE CAD/AI - a suite of cloud-based, AI-powered apps, marking the first step in its intelligent endoscopy ecosystem. These apps support clinicians in detecting, characterizing, and analyzing lesions throughout the upper and lower GI tract:
- CADDIE2: A cloud-based AI solution for colonoscopy that assists physicians in both detecting and characterizing colorectal polyps. Trained on a robust dataset, the system analyzes colonoscopy video frames to assist in identifying lesions, including high-risk types such as large polyps and hard-to-detect ones like sessile serrated lesions (SSLs).
- CADU3: A cloud-based AI solution designed to support the detection of dysplasia in patients with Barrett’s Esophagus. By identifying and highlighting the areas with the highest likelihood of dysplasia in endoscopy images, CADU aims to support physicians in making more accurate clinical decisions.
- SMARTIBD3: A cloud-based AI solution that supports the objective assessment of ulcerative colitis through MAYO scoring system. SMARTIBD can analyse the visual characteristic and provide information to aid the users and decrease subjectivity in the clinical decision-making process.
Comment from Professor Cesare Hassan in Gastroenterology from Humanitas University, Italy
“Cloud-based and AI-powered endoscopy solutions are transforming clinical practice. By enabling real-time decision support and standardized assessments, they help clinicians diagnose earlier and more accurately, streamline workflows, and improve patient outcomes. Endoscopy on the cloud opens the door for continuous innovation and broader access to advanced care.”
Comment from Keith Boettiger, Corporate Officer, Head of Gastrointestinal Solutions Division, Olympus Corporation
“With OLYSENSE, we are taking a meaningful step forward in helping patients receive earlier, more accurate diagnoses. Our goal is simple: to give clinicians the advanced tools that make it easier to find and assess challenging lesions early, make confident, informed decisions, and provide their patients with the best possible care.”
In Europe, CADDIE, CADU, and SMARTIBD each received CE Mark certification under the EU-MDR (July 2024), with CADDIE covering both computer-aided detection (CADe) and diagnosis (CADx) of suspected colorectal polyps.
In the United States, the CADDIE computer-aided detection device received Food and Drug Administration (FDA) 510(k) clearance (July 2024) to assist gastroenterologists in detecting suspected colorectal polyps during colonoscopy procedures. CADDIE is available in the US with only polyp detection and excludes polyp characterization features. SMARTIBD and CADU are not available in the US.
Through OLYSENSE, Olympus aims to integrate clinical and operational solutions, providing seamless data connectivity across the GI unit and integration with hospital systems — creating an intelligent endoscopy ecosystem.
As part of its long-term vision, Olympus is committed to expanding OLYSENSE, solution by solution, ensuring each new capability meets the evolving needs of clinicians worldwide.
1 For detailed information regarding instructions for use, indications, contraindications, warnings, and precautions, please consult the device manual.
2 CADDIE is available in the US with only polyp detection and excludes polyp characterization features.
3 SMARTIBD and CADU are not available in the US.
All company names and product names mentioned are trademarks or registered trademarks of their respective companies. All trademarks and registered trademarks are the property of their respective owners.
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide innovative solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit https://www.olympus-global.com/ and follow our global LinkedIn and X accounts.
Media contact:
Mail: Global-Public_Relations@olympus.com
Olympus Corp [TYO: 7733] [ADR: OLYMY] [STU: OLY1] [FRA: OLYS] https://www.olympus-global.com