THUNDERBEAT™ II features the versatility of three energy modes to support surgeons in adapting to challenging clinical conditions
TOKYO & HAMBURG, Oct 21, 2025 - (JCN Newswire) - Olympus Corporation (Olympus), a global MedTech company committed to making people’s lives healthier, safer and more fulfilling, today announced the launch of THUNDERBEAT™ II, the next generation hybrid energy device for soft tissue management, designed to deliver faster, more hemostatic dissection and large vessel transection1 in both laparoscopic and open surgery. THUNDERBEAT™ II will become commercially available in Europe starting October 2025, with a subsequent launch in Japan later this year. A global rollout, including the United States and other regions, will follow, subject to regulatory approvals including product registration and market clearance.
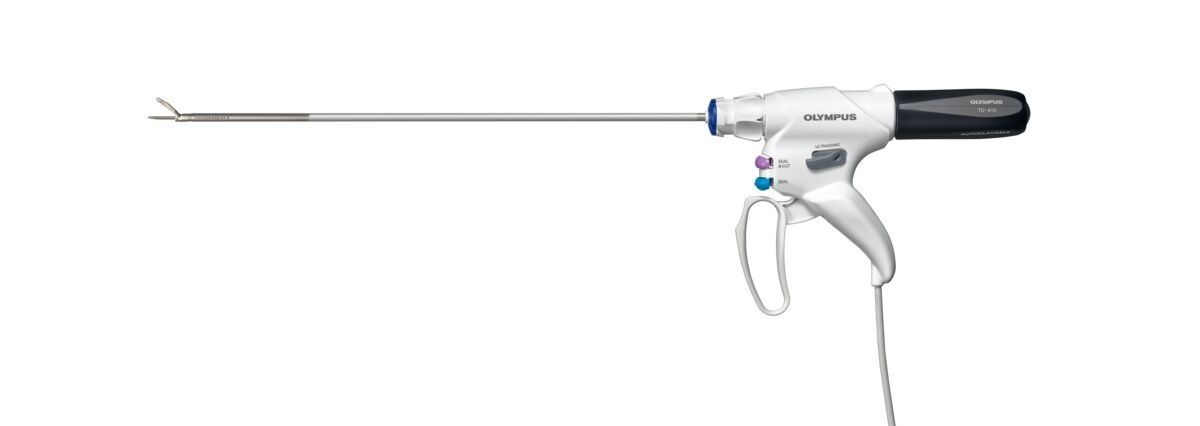 THUNDERBEAT II
THUNDERBEAT II
THUNDERBEAT II is part of Olympus’ THUNDERBEAT series, a line of pioneering hybrid energy devices designed for use in both laparoscopic and open surgery. Compared to the previous generation, THUNDERBEAT II delivers faster hemostatic dissection and more secure vessel sealing, featuring a refined probe design that minimizes thermal impact on surrounding tissue. Additionally, the versatility of three energy modes — including a new ultrasonic energy setting — supports surgeons in technically challenging procedures, enabling them to adapt to changing clinical conditions without interrupting the procedure to exchange instruments. THUNDERBEAT II offers surgeons even more features and greater versatility than ever before.
“Built to support surgeons in complex surgical procedures, THUNDERBEAT II underscores Olympus’ commitment to advancing hybrid energy technology. We are excited to enhance our surgical energy portfolio with its introduction, as it is designed to address surgeons’ needs and improve clinical outcomes” said Phil Roy, Surgical Deputy General Manager and Surgical Devices Global Business Unit Leader.
Three Energy Modes Deliver Enhanced Versatility
THUNDERBEAT II features three output modes: hybrid energy (SEAL & CUT Mode) for fast, precise hemostatic dissection compared to its previous model; advanced bipolar energy (SEAL Mode) for vessel pre-sealing and spot hemostasis; and newly added ultrasonic energy (ULTRASONIC Mode) for fine dissection in anatomical spaces when cutting without bipolar energy is required.
Refined Jaw Design with Thermal Shield Technology
The refined slim probe and jaw of THUNDERBEAT II support accurate and fine dissection while minimizing impact on surrounding tissue. In addition, the newly developed thermal shield at the distal tip slows heat transfer from the probe to the exterior surface of the jaw, reducing the risk of unintended heat damage to surrounding tissues and vessels.
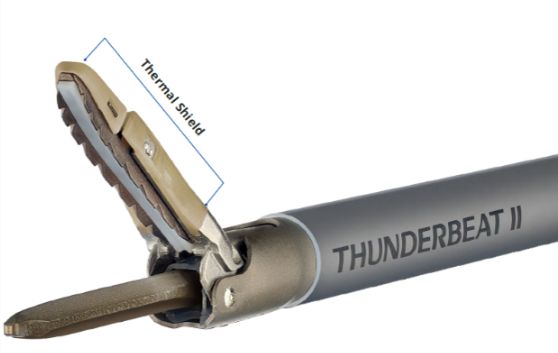 Thermal Shield
Thermal Shield
Ergonomic Handle Design with Cordless Transducer
The improved ergonomic handle reduces the grip force required to operate the jaws and provides audible and haptic feedback to confirm full closure, supporting surgeon comfort throughout the procedure. THUNDERBEAT II also introduces a cordless transducer that relocates the cable to the base of the handle, designed to give surgeons greater freedom of movement during complex procedures.
 Comparison of cable location (left: THUNDERBEAT Type S, right: THUNDERBEAT II)
Comparison of cable location (left: THUNDERBEAT Type S, right: THUNDERBEAT II)
1 up to 7mm
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide innovative solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit https://www.olympus-global.com/ and follow our global LinkedIn and X accounts.
Media contact:
Mail: Global-Public_Relations@olympus.com
Olympus Corp [TYO: 7733] [ADR: OLYMY] [STU: OLY1] [FRA: OLYS] https://www.olympus-global.com