Shanghai, Jun 23, 2025 - (ACN Newswire) - Hua Medicine ("the Company", stock code: 2552.HK) announced today that the Company presented the latest research results of dorzagliatin, its global first-in-class glucokinase activator (GKA), at the 85th Scientific Sessions of the American Diabetes Association (ADA). A preclinical animal study showed that the combination of dorzagliatin and sitagliptin, a DPP-4 inhibitor, improves blood glucose levels, promotes insulin secretion, and enhances GLP-1 secretion. The combination therapy was more effective than dorzagliatin alone. The blood chemistry analysis further indicates that the combination therapy has potential benefits for lipid lowering (especially LDL) (See Figure 1).
Dorzagliatin is the world's first glucokinase activator independently developed by Hua Medicine, aiming to restore the imbalanced blood glucose levels in patients with type 2 diabetes by repairing the impaired function and expression of glucokinase and enhancing glucose sensitivity in patients with type 2 diabetes. Sitagliptin, a DPP4 inhibitor, improves glucose control by blocking degradation of GLP-1. This study aims to assess the impact of long-term administration of dorzagliatin and the combination of dorzagliatin/sitagliptin on glucose homeostasis in high-fat diet-induced obesity/diabetes (DIO) mice.
In the study, the DIO mice were administered dorzagliatin at a dosage of 30 mg/Kg/day, or a combination of dorzagliatin (same dose) and sitagliptin (20mg/Kg/day) for 30 days. Mice on a standard diet served as controls. On day 30, the glucose levels were all reduced compared to pre-treatment, and the combination therapy was more effective than dorzagliatin alone. Dorzagliatin monotherapy promoted insulin and GLP-1 secretion, and the combination resulted in a further increase.
Blood biochemical analysis showed that drug treatment significantly improved glycated serum protein (GSP) in DIO mice. Combination therapy also improved low-density lipoprotein (LDL) levels. LDL transports cholesterol to arteries, and excessive LDL can cause arteriosclerosis, myocardial infarction, stroke, and peripheral arterial diseases. These results suggest that combination therapy has potential benefits reducing blood lipids (especially LDL) while restoring glucose homeostasis, exploring possibilities for clinical medication and new indication expansion.
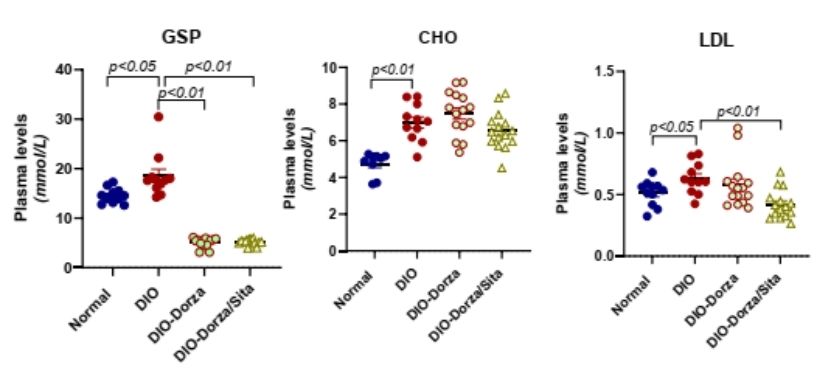
Figure 1 Blood Biochemical 2025 ADA 893-P
Another investigator-initiated real-world study was also presented at the ADA conference. This single-center prospective observational study aimed to evaluate the short-term efficacy and underlying mechanisms of dorzagliatin in T2DM patients. Interim analysis showed significant reductions in HbA1c levels, improvements in continuous glucose monitoring parameters, and protection of β-cell insulin secretion capacity as indicated by β-cell function indices (See Figure 2). The key analysis data are as follows:
HbA1c
- decreased significantly from 8.1±1.2% at baseline to 7.3±1.1% at 3 months
- sustained the reduction to 7.3±1.1% at 6 months (both p<0.0001).
CGM metrics
- TIR increased from 65.8±22.1% to 70.3±21.5% (p=0.047) at 6 months
- TAR decreased from 31.8±22.7% to 25.7±21.6% (p=0.033).
β-cell function index AUC_CP
- improved by 10.9% at 6 months (p<0.0001)
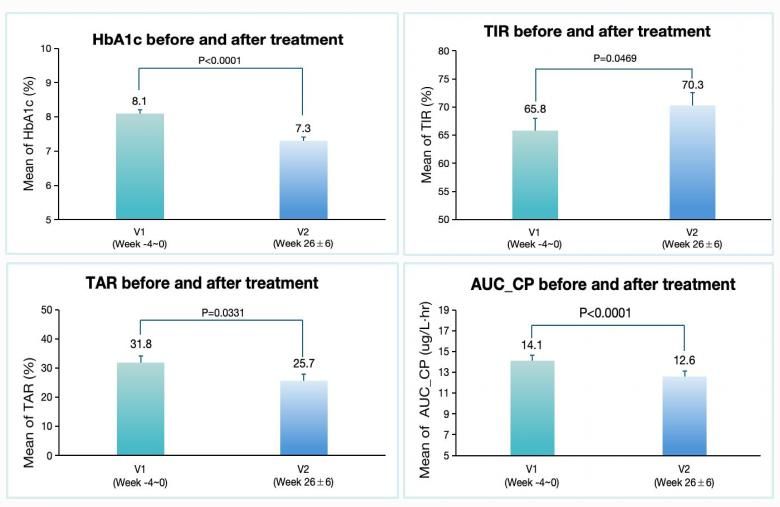
(Figure 2 improvements of glycated hemoglobin, CGM metrics, β-cell function from baseline to 6 months 2025ADA 2004-LB)
These real-world clinical application cases confirm that dorzagliatin, as a GKA, can improve glucose sensitivity, regulate blood glucose levels, and improves glycemic homeostasis and β-cell function in T2DM patients, supporting GKAs' unique therapeutic position in diabetes management, distinct from conventional hypoglycemic agents. Researchers will continue to evaluate the long-term efficacy of dorzagliatin and the long-term effects of pancreatic function preservation through follow-up.
Additionally, Hua Medicine was again invited to the official ADA TV channel during the ADA conference to present a series of films discussing the global diabetes management landscape and the clinical application value of dorzagliatin in various fields.
Hua Medicine will continue to conduct preclinical and clinical research, and through extensive cooperation with clinicians, further explore the clinical application potential of dorzagliatin to expand the Company's future product pipeline and disease areas.
About Hua Medicine
Hua Medicine (The “Company”) is an innovative drug development and commercialization company based in Shanghai, China, with companies in the United States and Hong Kong. Hua Medicine focuses on developing novel therapies for patients with unmet medical needs worldwide. Based on global resources, Hua Medicine teams up with global high-calibre people to develop breakthrough technologies and products, which contribute to innovation in diabetes care. Hua Medicine's cornerstone product HuaTangNing (dorzagliatin tablets), targets the glucose sensor glucokinase, restores glucose sensitivity in T2D patients, and stabilizes imbalances in blood glucose levels in patients. HuaTangNing was approved by the National Medical Products Administration (NMPA) of China on September 30th, 2022. It can be used alone or in combination with metformin for adult T2D patients. For patients with chronic kidney disease (CKD), no dose adjustment is required. It is an oral hypoglycemic drug that can be used for patients with Type 2 diabetes with renal function impairment.
For more information
Hua Medicine
Website: www.huamedicine.com
Investors
Website: ir@huamedicine.com
Media
Website: pr@huamedicine.com