|
Hua Medicine Successfully Completes SEED (HMM0301), Dorzagliatin Phase III Monotherapy Trial
SHANGHAI, CHINA, Jun 18, 2020 - (ACN Newswire) - Hua Medicine (2552.HK), a leading innovative drug development company focused on developing novel therapies for the treatment of diabetes, today announced topline results from SEED (also known as HMM0301), the first Phase III trial with dorzagliatin. Dorzagliatin is a first-in-class glucokinase activator administered orally, twice daily. The 52-week trial investigated the efficacy and safety of 75mg BID dorzagliatin in 463 patients with Type 2 diabetes, with an initial 24-week double blinded, placebo-controlled treatment, followed by an open label 28-week treatment. The primary efficacy and safety endpoints were evaluated at 24 weeks.
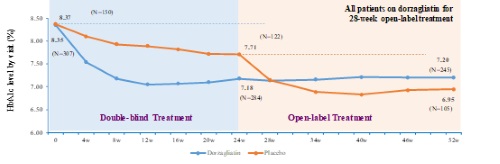 | | Figure 1 |
 | | HbA1c Reduction *p<0.001 compared with baseline at 52 week |
In November 2019, Hua Medicine announced the trial had achieved its primary efficacy and safety endpoints over the initial 24-week double blinded period. For the 52-week treatment period, the efficacy and safety profiles were sustained based on the topline data analysis. During the 28-week open-label period, patients initially receiving a placebo (i.e., the placebo group) were administered dorzagliatin for the first time. Figure 1 below illustrates the efficacy (as measured by HbA1c reduction) for the two-cohort groups for the entire 52-week period.
In the 28-week open-label treatment period, dorzagliatin continued to exhibit a safe and well-tolerated clinical profile. A safety analysis based on study safety population demonstrated that dorzagliatin was well tolerated and had a good safety profile. The incidence of adverse events was similar between the dorzagliatin-treated and placebo groups. There was less than 1% hypoglycemia with blood glucose < 3 mmol/L during the 52-week treatment period. During the 28-week open-label treatment, patients also saw a continued reduction of insulin resistance (insulin resistance is the hallmark of Type 2 diabetes).
"We are incredibly proud of our accomplishment. The Hua Medicine team and our partners have worked closely together over the last decade to advance the development of dorzagliatin," said Dr. Li Chen, CEO and founder of Hua Medicine. "With the successful completion of SEED, we become the first company globally to advance a glucokinase activator through clinical development. This is an incredible achievement for the Hua Medicine team, Chinese investigators, Hua's partners and supporters, and most importantly, for Type 2 diabetes patients globally." On Sunday, June 14th, 2020, Dr Chen presented more comprehensive data of the 24-week double blinded, placebo-controlled period of SEED study at the ADA 2020 80th Scientific Sessions. In addition to reduced glucose levels, the data presented at the ADA indicated improved beta cell function for the dorzagliatin-treatment group (as measured by the clinically meaningful biomarker HOMA2-beta). In contrast, the placebo group experienced reduced beta cell function during the same period. Dr. Chen added: "Hua Medicine will continue its efforts to develop a potential disease modifying therapy to treat diabetes."
SEED (Efficacy and Safety Evaluation of Dorzagliatin) study design
SEED is a randomized, double-blind, placebo-controlled Phase III study in 463 drug naive type 2 diabetes patients. Patients are treated with twice-daily doses of dorzagliatin (75 mg) or placebo, randomized 2:1. The clinical study evaluated the efficacy and safety of dorzagliatin during 24 weeks of double-blinded treatment, followed by a subsequent 28-week open-label treatment period, for a total of 52 weeks. During the 28-week open-label period, both patient groups were treated with twice-daily doses of dorzagliatin (75 mg). The trial was conducted in compliance with the guidelines of the Chinese Society of Endocrinology, which require physicians to educate patients and strictly enforce improved exercise and dietary control, as well as continuous self-monitoring, in treating Type 2 diabetes. The trial was conducted at 40 clinical sites across China led by Professor Dalong Zhu, President of the Chinese Diabetes Society.
About Dorzagliatin
Dorzagliatin is an investigational first-in-class, dual-acting glucokinase activator, designed to control the progressive degenerative nature of diabetes by restoring glucose homeostasis in patients with Type 2 Diabetes. By addressing the defect of the glucose sensor function of glucokinase, dorzagliatin has the potential to restore the impaired glucose homeostasis state of patients with Type 2 Diabetes and serve as a first-line standard-of-care therapy for the treatment of the disease, or as a cornerstone therapy when taken in combination with currently approved anti-diabetes drugs.
About Hua Medicine
Hua is a leading, clinical-stage innovative drug development company in China focused on developing novel therapies for the treatment of diabetes. Founded by an experienced group of entrepreneurs and international investment firms, Hua advanced a first-in-class oral drug for the treatment of Type 2 Diabetes into NDA-enabling stage and is currently evaluating the therapy in adults with diabetes in two Phase III trials in China and various earlier stage clinical trials in China and the United States. Dorzagliatin has achieved its first primary endpoint in a Phase III monotherapy trial. The Company has initiated product life-cycle management studies of this novel diabetes therapy and advanced its use in personalized diabetes care. Hua Medicine is working closely with disease experts and regulatory agencies in China and across the world to advance diabetes care solutions for patients worldwide.
For more information
Hua Medicine
Website: www.huamedicine.com
Investors
Email: ir@huamedicine.com
Media
Email: pr@huamedicine.com
Source: Hua Medicine
Sectors: Daily Finance, Daily News, BioTech
Copyright ©2026 ACN Newswire. All rights reserved. A division of Asia Corporate News Network. |
Latest Release

MHI and SoftBank Corp. Collaborate to Adapt AI-RAN Network Architecture for Edge Data Centers
Mar 02, 2026 19:13 JST
| 
DOCOMO and NEC Launch Japan's First Commercial 5G Core on AWS, Built with World's First AI-automated Network Construction Technology
Mar 02, 2026 16:34 JST
| 
Fujitsu supports sustainable growth for retailers with data and AI through Uvance for Retail
Mar 02, 2026 11:55 JST
| 
WELIREG(R) (belzutifan) Plus LENVIMA(R) (lenvatinib) Reduced the Risk of Disease Progression or Death by 30% Compared to Cabozantinib in Certain Previously Treated Patients With Advanced Renal Cell Carcinoma (RCC)
Mar 02, 2026 09:49 JST
| 
NEC Strengthens Multi-Vendor Optical Network Solutions with New TIP-Certified Phoenix Hardware Lineup
Feb 27, 2026 18:50 JST
| 
NEC Demonstrates Agentic AI-Driven Autonomous Network Operations in Collaboration with AWS
Feb 27, 2026 17:31 JST
| 
Mitsubishi Heavy Industries Compressor Acquires Swiss Rotating Equipment Maintenance Company AST Turbo AG
Feb 27, 2026 17:23 JST
| 
NEC Strengthens Its Edge Portfolio for the 6G Era, Enabling Efficient Edge Deployment and Integration with Cloud Services
Feb 27, 2026 12:24 JST
| 
Fujitsu POS solution enhances customer experience at Hankyu Hanshin Department Stores
Feb 27, 2026 12:03 JST
| 
Mitomo Semicon Engineering to Change Company Name
Feb 26, 2026 22:00 JST
| 
The University of Tokyo, NTT, and NEC demonstrate real-time augmented reality assistance made possible by integrating three newly proposed technologies on a 6G/IOWN platform to realize the widespread use of AI agents supporting safety and security
Feb 26, 2026 19:01 JST
| 
NEC and Nokia to Expand Eletronet's Optical Fiber Network in Brazil by 50%
Feb 26, 2026 18:51 JST
| 
Everest Medicines Shareholders Pass Resolutions Including Commercialization Service Agreement at EGM
Feb 25, 2026 21:59 JST
| 
Toyota Launches New bZ4X Touring BEV Focused on Driving Performance and Spaciousness in Japan
Feb 25, 2026 21:58 JST
| 
DOCOMO and Keio University Demonstrate World's First Stable, High-Fidelity Robot Teleoperation via Commercial 5G Using Low-Latency Slicing
Feb 25, 2026 21:01 JST
| 
DOCOMO Successfully Demonstrates AI Application Operation Using Virtualized Radio Access Network (vRAN) Infrastructure
Feb 25, 2026 20:50 JST
| 
DOCOMO Begins Commercial Deployment of Agentic AI System for Network Maintenance Using One of the World's Largest Datasets
Feb 25, 2026 20:40 JST
| 
NEC Implements AI Code Review Service "Metabob," Reducing Technical Verification Time by Up to 66%
Feb 25, 2026 18:00 JST
| 
Beisia Automates Supermarket Refrigerator Temperature Monitoring and Recording with Fujitsu's IoT Visualization Solution
Feb 25, 2026 12:00 JST
| 
Hong Kong Tech Delegation Heading for Market Expansion at Mobile World Congress 2026
Feb 25, 2026 07:00 JST
|
More Latest Release >>
|