Nov 30, 2022 10:21 JST
 Source: Eisai
Source: Eisai
|
|
|
Eisai Presents Full Results of Lecanemab Phase 3 Confirmatory Clarity Ad Study for Early Alzheimer's Disease at Clinical Trials on Alzheimer's Disease (CTAD) Conference
TOKYO, Nov 30, 2022 - (JCN Newswire) - Eisai Co., Ltd. and Biogen Inc. announced today that the results from Eisai's large global Phase 3 confirmatory Clarity AD clinical study of lecanemab (development code: BAN2401), an investigational anti-amyloid beta (Abeta) protofibril antibody for the treatment of mild cognitive impairment (MCI) due to Alzheimer's disease (AD) and mild AD (collectively known as early AD) with confirmed presence of amyloid pathology in the brain, were presented at the 2022 Clinical Trials on Alzheimer's Disease (CTAD) conference, in San Francisco, California and virtually.
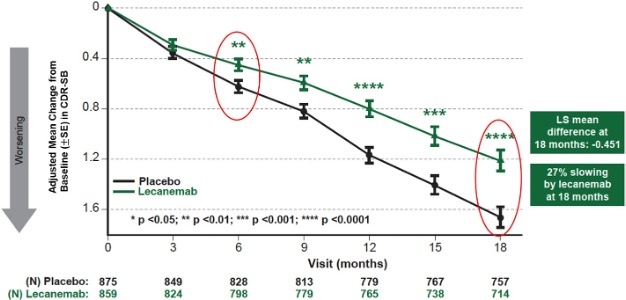 | | Figure 1: CDR-SB as Primary endpoint change (18 months) |
Summary of Presentations in the Scientific Session featuring Lecanemab at CTAD
Design of Clarity AD Study
Eisai's Clarity AD was a global confirmatory Phase 3 placebo-controlled, double-blind, parallel-group, randomized study in 1,795 people with early AD (lecanemab group: 898 placebo group: 897) at 235 sites in North America, Europe, and Asia. The participants were randomized 1:1 to receive either placebo or lecanemab 10-mg/kg IV biweekly, and the randomization was stratified according to clinical subgroup (MCI due to AD or mild AD), presence or absence of concomitant approved AD symptomatic medication at baseline (e.g., acetylcholinesterase inhibitors, memantine, or both), ApoE4 status and geographical region. Eligibility criteria allowed patients with a broad range of comorbidities/comedications, including but not limited to hypertension, diabetes, heart disease, obesity, renal disease and anti-coagulants. As a result of Eisai's recruitment strategy of diversity in the Clarity AD study, 4.5% and 22.5% of the randomized participants in the U.S. were Black and Hispanic, respectively.
The primary endpoint was change from baseline at 18 months in the CDR-SB1 (Clinical Dementia Rating Sum of Boxes), the global cognitive and functional scale, and key secondary endpoints were the change from baseline at 18 months in amyloid Positron Emission Tomography (PET) using Centiloids, AD Assessment Scale - Cognitive Subscale 14 (ADAS-Cog142), AD Composite Score (ADCOMS3) and AD Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS MCI-ADL4). In addition, longitudinal changes in brain tau pathology as measured by tau PET (n=257) and cerebrospinal fluid (CSF) biomarkers of AD pathology (n=281) were evaluated in optional sub-studies.
Efficacy Results of Clarity AD
Mean change of CDR-SB from baseline at 18 months as the primary endpoint was 1.21 and 1.66 for lecanemab and placebo groups, respectively. Lecanemab treatment resulted in highly statistically significant results, reducing clinical decline on the global cognitive and functional scale, compared with placebo at 18 months by -0.45 (95% Confidence Interval (CI): -0.67, -0.23; P=0.00005), representing a 27% slowing of decline. Starting as early as six months (difference: -0.17 [95% CI: -0.29, -0.05]; P<0.01), and increasing in absolute difference over time across all time points every 3 months, the treatment showed highly statistically significant changes in CDR-SB from baseline compared to placebo (all p-values are less than 0.01) (Figure 1).
All key secondary endpoints also showed highly statistically significant results compared with placebo (P<0.001). In the amyloid PET sub-study, treatment with lecanemab showed statistically significant reduction in amyloid plaque burden at all timepoints starting at 3 months. Mean change in Centiloids at 18 months was -55.5 and 3.6 for lecanemab and placebo groups, respectively (mean difference: -59.1 [95%CI: -62.6, -55.6]; P<0.00001). Lecanemab slowed decline of cognitive function by 26% on ADAS-Cog14 at 18 months (mean difference: -1.44 [95%CI: -2.27, -0.61]; P=0.00065). In the ADCOMS assessment, lecanemab slowed disease progression by 24% at 18 months (mean difference: -0.050 [95% CI: -0.074, -0.027; P=0.00002]). Lecanemab slowed decline of activities of daily living by 37% on ADCS MCI-ADL at 18 months (mean difference: 2.016 [95%CI: 1.208, 2.823]; P<0.00001). In addition, the primary stratified analysis showed consistent results in CDR-SB, ADAS-Cog14 and ADCS MCI-ADL at 18 months of treatment with lecanemab in all subgroups of disease stage (MCI due to AD or mild AD), ApoE4 status (non-carriers, carriers), presence or absence of concomitant approved AD symptomatic medication, and region (North America, Asia, Europe).
For more information, visit www.eisai.com/news/2022/pdf/enews202285pdf.pdf.
Source: Eisai
Sectors: BioTech
Copyright ©2024 JCN Newswire. All rights reserved. A division of Japan Corporate News Network. |
Latest Release

JCB enables JCB Contactless acceptance at Taichung MRT in Taiwan
Apr 26, 2024 10:00 JST
| 
Mazda Production and Sales Results for March 2024 and for April 2023 through March 2024
Apr 25, 2024 18:21 JST
| 
MHI Begins Operation of SOEC Test Module the Next-Generation High-Efficiency Hydrogen Production Technology at Takasago Hydrogen Park
Apr 25, 2024 17:45 JST
| 
GAC Honda to Begin Sales of All-new e:NP2, the Second Model of e:N Series
Apr 25, 2024 16:50 JST
| 
Toyota Exhibiting at Beijing Motor Show 2024
Apr 25, 2024 16:25 JST
| 
Honda Reaches Basic Agreement with Asahi Kasei on Collaboration for Production of Battery Separators for Automotive Batteries in Canada
Apr 25, 2024 11:10 JST
| 
UNIQLO Sponsors KAWS + Warhol Exhibition Tour, Starting in Pittsburgh
Apr 25, 2024 09:00 JST
| 
Mitsubishi Power Begins Commercial Operation of Seventh M701JAC Gas Turbine in Thailand GTCC Project; Achieves 75,000 AOH To-Date
Apr 24, 2024 17:19 JST
| 
MC and Denka Sign J/V Agreement in Fullerene Business
Apr 24, 2024 17:02 JST
| 
Mitsubishi Motors Posts Record Sales in the Philippines in FY2023
Apr 24, 2024 13:56 JST
| 
NEC Develops High-speed Generative AI Large Language Models (LLM) with World-class Performance
Apr 24, 2024 13:25 JST
| 
Fujitsu SX Survey reveals key success factors for sustainability
Apr 23, 2024 10:25 JST
| 
Fujitsu and METRON collaborate to drive ESG success: slashing energy costs, boosting productivity with new manufacturing industry solutions
Apr 22, 2024 16:09 JST
| 
NEC Strengthens Commitment to Space Industry with Investment in Seraphim Space Venture Fund II
Apr 22, 2024 15:09 JST
| 
Soft Space Launches the First and Only JCB Payment Gateway in Malaysia
Apr 22, 2024 15:00 JST
| 
TOYOTA GAZOO Racing takes a one-two in Croatian thriller
Apr 22, 2024 10:47 JST
| 
First-ever Mazda CX-80 Crossover SUV Unveiled in Europe
Apr 19, 2024 13:50 JST
| 
Fujitsu develops technology to convert corporate digital identity credentials, enabling participation of non-European companies in European data spaces
Apr 19, 2024 10:17 JST
| 
Mitsubishi Heavy Industries and NGK to Jointly Develop Hydrogen Purification System from Ammonia Cracking Gas
Apr 18, 2024 17:01 JST
| 
Toyota Launches All-New Land Cruiser "250" Series in Japan
Apr 18, 2024 13:39 JST
|
More Latest Release >>
|