|
Fujitsu Launches Japan's First System for Automatically Generating Electronic Submission Data for Pharmaceuticals
tsClinical for SDTM Automation contributes to new drug development in Japan by supporting and promoting the use of electronic data application
TOKYO, Oct 30, 2019 - (JCN Newswire) - Fujitsu Limited today announced that it has developed tsClinical for SDTM Automation, a solution for pharmaceutical companies that automatically converts electronic study data to the standardized Study Data Tabulation Model (SDTM) format. SDTM Automation is available for pharmaceutical companies in Japan as of today.
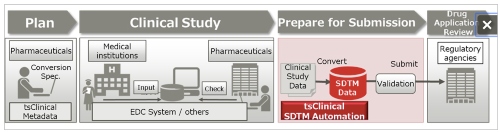 | | Process from clinical trial planning to drug approval |
 | |
This solution can automatically convert the submission data to the standard SDTM format that pharmaceutical companies must submit to regulatory agencies when applying for approval of a new drug, which is promoted by the Clinical Data Interchange Standards Consortium (CDISC), an organization that establishes global standards for clinical study data. By automatically converting the submission data, this solution prevents issues caused by human error or misunderstanding of the standard in manual conversion, reducing the cost required to convert electronic study data to SDTM format and improving the quality of the data.
The Pharmaceuticals and Medical Devices Agency (PMDA)(1), a regulatory agency in Japan, will require all applications to be submitted electronically in SDTM format starting in April 2020. By offering this solution, Fujitsu is providing world-leading support for the automatic generation of SDTM data (patent pending). This will quickly resolve issues that many pharmaceutical companies may face, such as ensuring the quality of SDTM data, securing human resources to generate the data, and minimizing data generation costs, while also supporting PMDA in their efforts to promote the use of clinical data, contributing to new drug development in Japan.
Background
PMDA, which evaluates and approves pharmaceuticals such as drugs submitted by pharmaceutical companies, is making the submission of SDTM data mandatory beginning in April 2020, to promote the use of clinical trial data for new drug development. In order to meet the requirements, pharmaceutical companies and CROs (Contract Research Organization) have an urgent need for systems and processes to generate the SDTM data.
Currently, the generation of SDTM data has not been systematized, and the relevant personnel at pharmaceutical companies manually create conversion specifications and programs for each clinical trial in order to convert data that was captured by electronic data capture (EDC) systems or other systems in a variety of formats. The manual process means that human errors can occur, and the cost required to generate the SDTM data can reach around 10 million yen per trial(2). In addition, there have been cases of various interpretations of the SDTM guidelines(3), indicating risks that work would have to be redone, while companies have also faced difficulty in securing personnel who understand the standard. CROs have also been unable to secure human resources to meet numerous requests from pharmaceutical companies, raising concerns that drug submission by Japanese pharmaceutical companies may be delayed, which would affect the society as a whole.
Major Features of the Solution
1. Improved application data quality and shorter submission preparation times through unified management of conversion specification documents
Because this solution makes it possible for each pharmaceutical company to set up and manage the conversion specification documents necessary when generating SDTM data within clinical Metadata, the clinical trial metadata management system offered by Fujitsu, it will be possible to maintain consistency throughout the SDTM data generation process. This helps to prevent errors due to inconsistencies in different employees' understanding of the standard or variation in inputs, helping to control the quality of the SDTM data. In addition, because SDTM data can be generated without programming, the previous need to manually create a conversion program, debug it, and then check the data after the program is run can be eliminated, shortening preparation times for submission.
2. Reduced labor and SDTM data generation costs through automation
Because conversion specification documents for each trial can be generated in the system based on a standardized conversion specification document for each pharmaceutical company, this solution reduces the burden of creating conversion specification documents. In addition, because this solution includes functionality for checking data consistency when generating conversion specification documents, it can prevent errors and improve efficiency in managing data generation. Moreover, the system can also track version numbers and logs, including logs of who generated what data at what times, for both conversion specification documents and SDTM data, including the clinical trial data it is based on. The system can thus reliably ensure the traceability required in the pharmaceutical industry, contributing to greater efficiency in responding to inquiries from regulatory agencies. These features reduce operating costs when applying for drug approval, from planning a clinical trial to preparing the data submission.
3. Support for a wide range of data formats from different types of data collection systems, including EDC systems
This solution is available as an optional add-on for users of clinical DDworks21/EDC plus, Fujitsu's case data collection system for clinical trials. It also supports data collected with EDC systems from other companies and data collected by outside institutions that conduct clinical tests(4). This means that it is capable of generating SDTM data regardless of the customers'' different collection systems.
Sales Target
100 clinical trials by the end of fiscal 2020.
Future Plans
Going forward, Fujitsu will expand the system's functionality, including automatic generation of conversion specification documents when generating SDTM data. This way, the company will contribute to greater efficiencies in drug submission operations in Japan and improved trial success rates, while also growing Fujitsu's business in the field of clinical trials.
(1) Pharmaceuticals and Medical Devices Agency (PMDA)
An independent agency managed by the Ministry of Health, Labour and Welfare. The institution provides guidance and oversight for the entire system that ensures the quality, effectiveness, and safety of drugs and medical devices, from prior to the start of clinical trials through approval, including the management of the approval process. It also collects, analyzes, and publishes information relating to the safety of drugs and medical devices after the commercial sale begins.
(2) Around 10 million yen per trial
As of October 4, 2019, according to Fujitsu's own investigations.
(3) SDTM guidelines
Guidelines for those generating SDTM data (https://www.cdisc.org/)
(4) Data collected with EDC systems from other companies and data collected by outside institutions that conduct clinical tests
Requires the deployment of tsClinical Metadata. In addition, this system does not support all products from other companies. For more information on supported systems, please see the product website.
Contact:Fujitsu Limited
Public and Investor Relations
Tel: +81-3-3215-5259
URL: www.fujitsu.com/global/news/contacts/
Source: Fujitsu Ltd
Sectors: Enterprise IT, Healthcare & Pharm
Copyright ©2025 JCN Newswire. All rights reserved. A division of Japan Corporate News Network. |
Latest Release

SAKENOVA: 28-Year-Old Master Brewer Pioneers AI-Driven Sake Revolution, Achieving 40% Cost Reduction While Winning International Gold Medals
Dec 15, 2025 23:00 JST
| 
NEC Provides Vehicle Management Equipment for Autonomous Driving at Tokyo International Airport
Dec 15, 2025 19:41 JST
| 
NEC and AEROTHAI Elevate Air Traffic Safety with Advanced Time Sync Solutions from Adtran Oscilloquartz
Dec 15, 2025 19:04 JST
| 
Olympus Triples Venture Capital Fund Investment to Strengthen MedTech Leadership
Dec 15, 2025 08:30 JST
| 
HKTDC 4Q25 Export Confidence Index: 2026 Hong Kong Export Growth of 8-9%, Sustained AI product demand lays solid foundation for future expansion
Dec 12, 2025 23:15 JST
| 
Honda to Launch New Heritage Service Business
Dec 12, 2025 20:31 JST
| 
Bank Mandiri Enhances Digital Resiliency for Financial Trading in Indonesia through Advanced Time Sync Solutions with NEC and Adtran Oscilloquartz
Dec 12, 2025 19:59 JST
| 
NEC and ClimateAi sign a Memorandum of Understanding (MoU) for business co-creation to enhance climate resilience across various industries
Dec 12, 2025 18:13 JST
| 
Anime Tokyo Station: A Series of Linked Events to Be Held in Conjunction with the Anniversary Joint Exhibition
Dec 12, 2025 11:00 JST
| 
Eisai Rated "A", the Highest Rating by CDP in Both Climate Change and Water Security Categories for the Second Consecutive Year
Dec 11, 2025 19:41 JST
| 
NEC Named to CDP's "A List" for Climate Change and Water Security for the Seventh Consecutive Year
Dec 11, 2025 19:00 JST
| 
MHI Successfully Produces Hydrogen at Its Ammonia Cracking Pilot Plant Using Steam Heating
Dec 10, 2025 18:14 JST
| 
NEC Presents Phase I Results for Personalized AI-Powered Oral Cancer Vaccine NECVAX-NEO1 at ESMO Immuno-Oncology Congress 2025
Dec 10, 2025 12:00 JST
| 
"LEQEMBI(R)" (lecanemab) for the Treatment of Early Alzheimer's Disease Included in China's Commercial Insurance Innovative Drug List
Dec 09, 2025 18:51 JST
| 
NEC Wins TIP MUST Silver Badge with Advanced Network Operating System for Open Optical Transponders
Dec 09, 2025 13:39 JST
| 
MHI and Worley to Deliver Full-Scale Carbon Capture Facility for Heidelberg Materials UK's Padeswood Cement Works
Dec 08, 2025 17:00 JST
| 
Honda Technical Support for Red Bull Powertrains in F1 Racing Concludes
Dec 08, 2025 16:34 JST
| 
MHIET U.S. Subsidiary's Franklin Plant Celebrates 10th Anniversary
Dec 08, 2025 16:03 JST
| 
Lexus World Premieres Lexus LFA Concept BEV Sports Car
Dec 05, 2025 19:13 JST
| 
TOYOTA GAZOO Racing World Premieres GR GT & GR GT3
Dec 05, 2025 18:58 JST
|
More Latest Release >>
|